AR-701 (COVID-19 mAb)
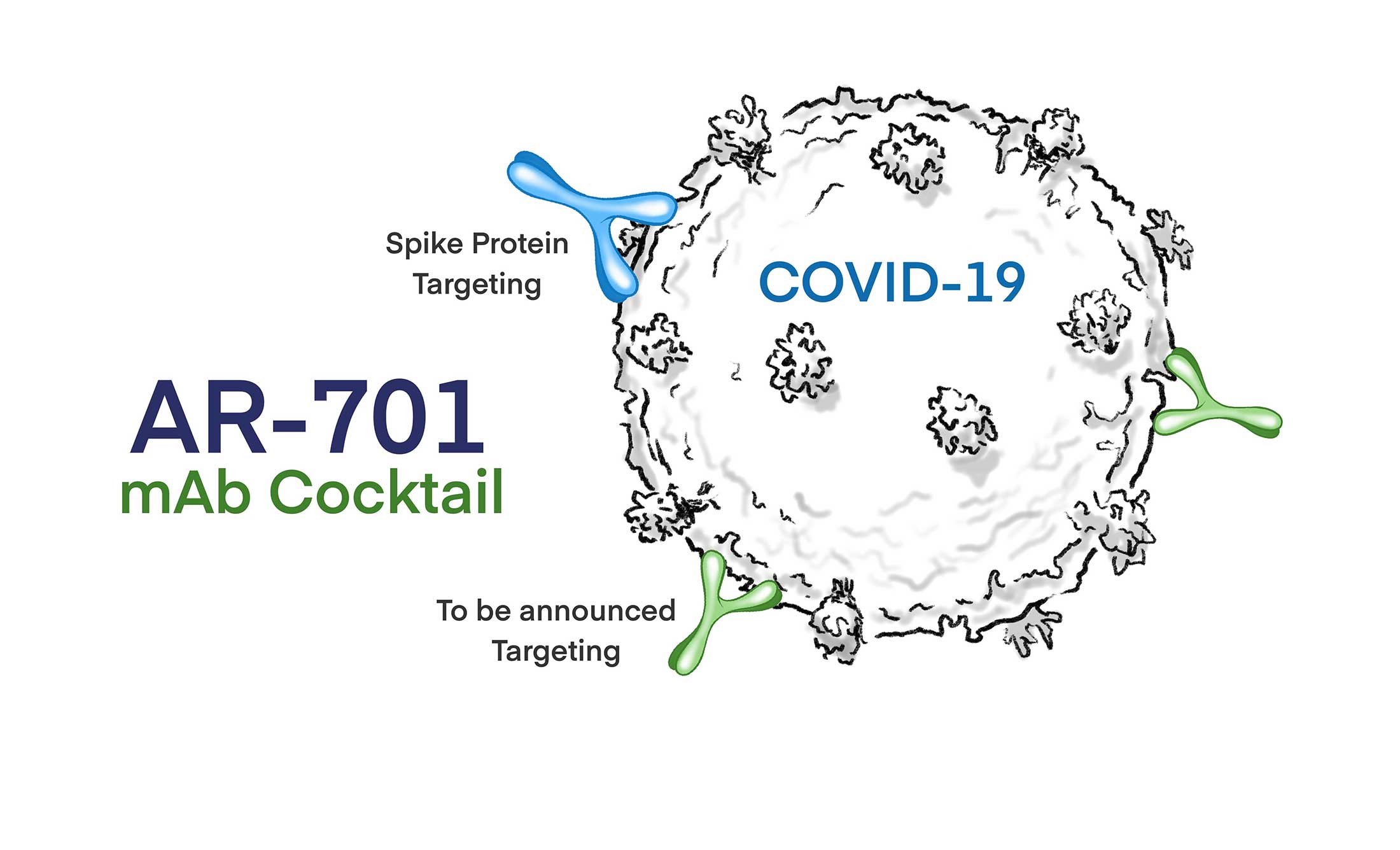
Human mAb Cocktail to SARS-CoV-2 Virus
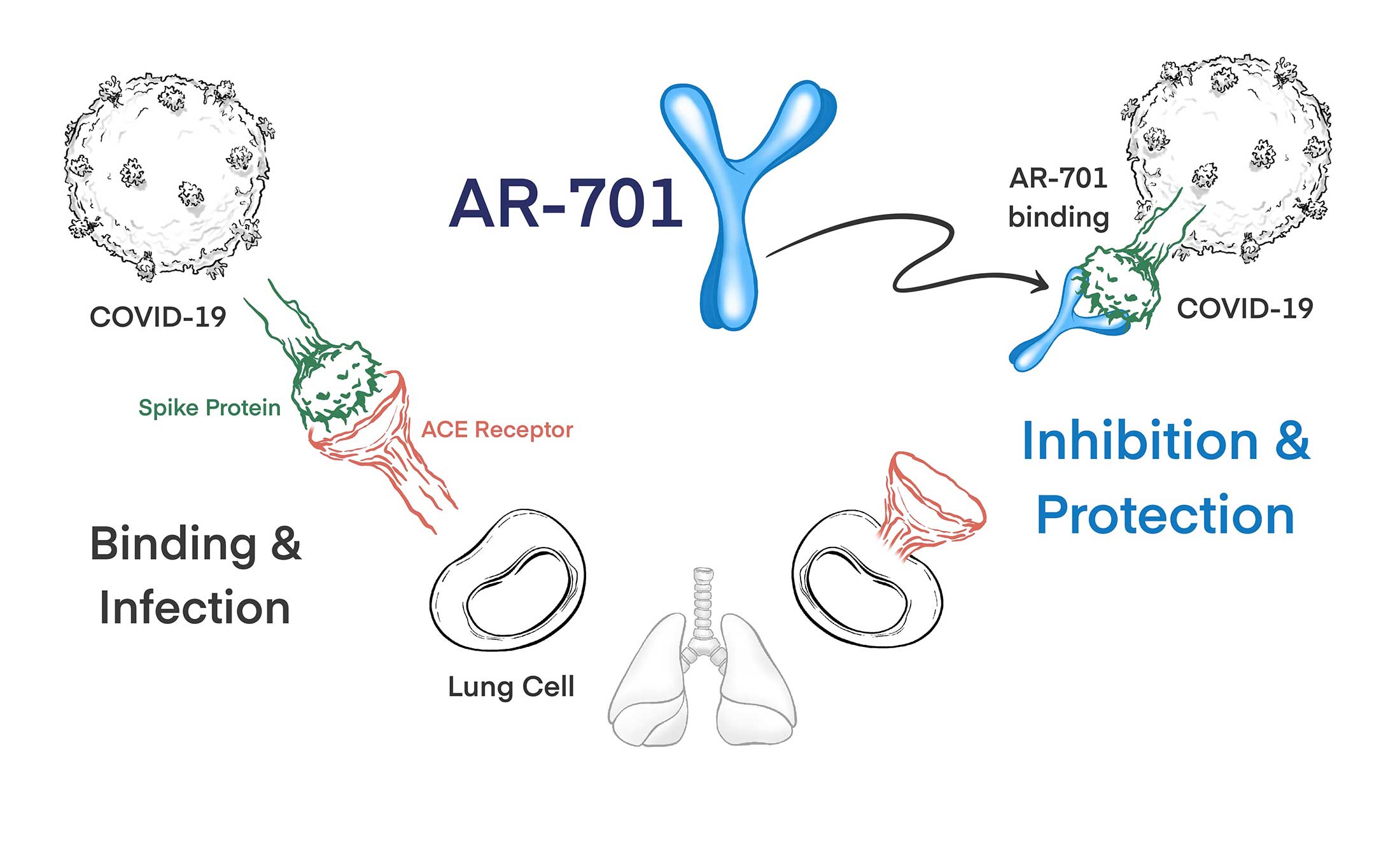
Development of safe and effective vaccines and therapeutics to prevent and treat SARS-CoV-2 infection (COVID-19) is a global urgency. Preliminary clinical studies on COVID-19 convalescent plasma and monoclonal antibodies have suggested the potential clinical benefits of using antibodies as prophylaxis or therapeutic treatments.
AR-701 was identified by screening the blood of COVID-19 convalescent of patients in U.S. and EU using Aridis’ ⅄PEXTM platform technology.
AR-701 is comprised of multiple fully human IgG1s monoclonal antibodies directed at conserved regions of the SARs-CoV-2 envelope proteins. AR-701 mAbs are designed to maintain broad coverage of SARs-CoV-2, including recently reported variants of SARS-CoV-2 such as the D614G variant, possible future variants of SARS-CoV-2.
Furthermore, AR-701 mAbs have been engineered for prolonging the duration of protection well beyond that afforded by typical mAbs. AR-701 is designed to block the binding and propagation of infection by SARS-CoV-2 of human lung cells.